Schedule a Visit
Complete the form to schedule a visit.
Bloom Energy has begun generating hydrogen from the world’s largest solid oxide electrolyzer installation at NASA’s Ames Research Center, the historic Moffett Field research facility in Mountain View, Calif. This high-temperature, high-efficiency unit produces 20-25% more hydrogen per megawatt (MW) than commercially demonstrated lower-temperature electrolyzers such as proton electrolyte membrane (PEM) or alkaline.
This hydrogen electrolyzer demonstration showcases the maturity, efficiency and commercial readiness of Bloom’s solid oxide technology for large-scale, clean hydrogen production. The 4 MW Bloom Electrolyzer™, delivering the equivalent of over 2.4 metric tonnes per day of hydrogen output, was built, installed and operationalized in a span of two months to demonstrate the speed and ease of deployment.
Hydrogen is critically important for decarbonizing the energy economy, while creating a sustainable hydrogen economy that reduces greenhouse gas emissions. Solid oxide electrolysis reduces the cost of producing hydrogen fuel.
The cost of producing hydrogen fuel via electrolysis is driven primarily by a single variable: the cost of electricity. Because electricity costs have the biggest impact on the financial viability of clean hydrogen, it is imperative that hydrogen electrolyzers consume as little electricity as possible. Researchers at Idaho National Laboratory (INL) have been conducting a variety of tests on Bloom Energy’s solid oxide electrolyzer at the Dynamic Energy Testing and Integration Laboratory.
Running at high temperatures and high availability, the pilot results reveal the Bloom Electrolyzer is producing hydrogen at 37.5 kWh per kilogram of hydrogen at the system level. Alternative efficient electrolyzer technologies, such as PEM or Alkaline, consume as much as 52 – 54 kWh per kilogram of hydrogen produced.
Learn more about the impact of efficiency on system economics and achieving decarbonization by contacting us.
Our solid oxide electrolyzer technology can be paired with renewable energy sources to create clean, low-cost green hydrogen with industry-leading efficiency.
Our solid oxide electrolyzer is built on the same platform as our Energy Server. The scale, experience, reliability and manufacturing capabilities that went into deploying over 1 GW of solid oxide fuel cells directly translate to our solid oxide electrolyzer.
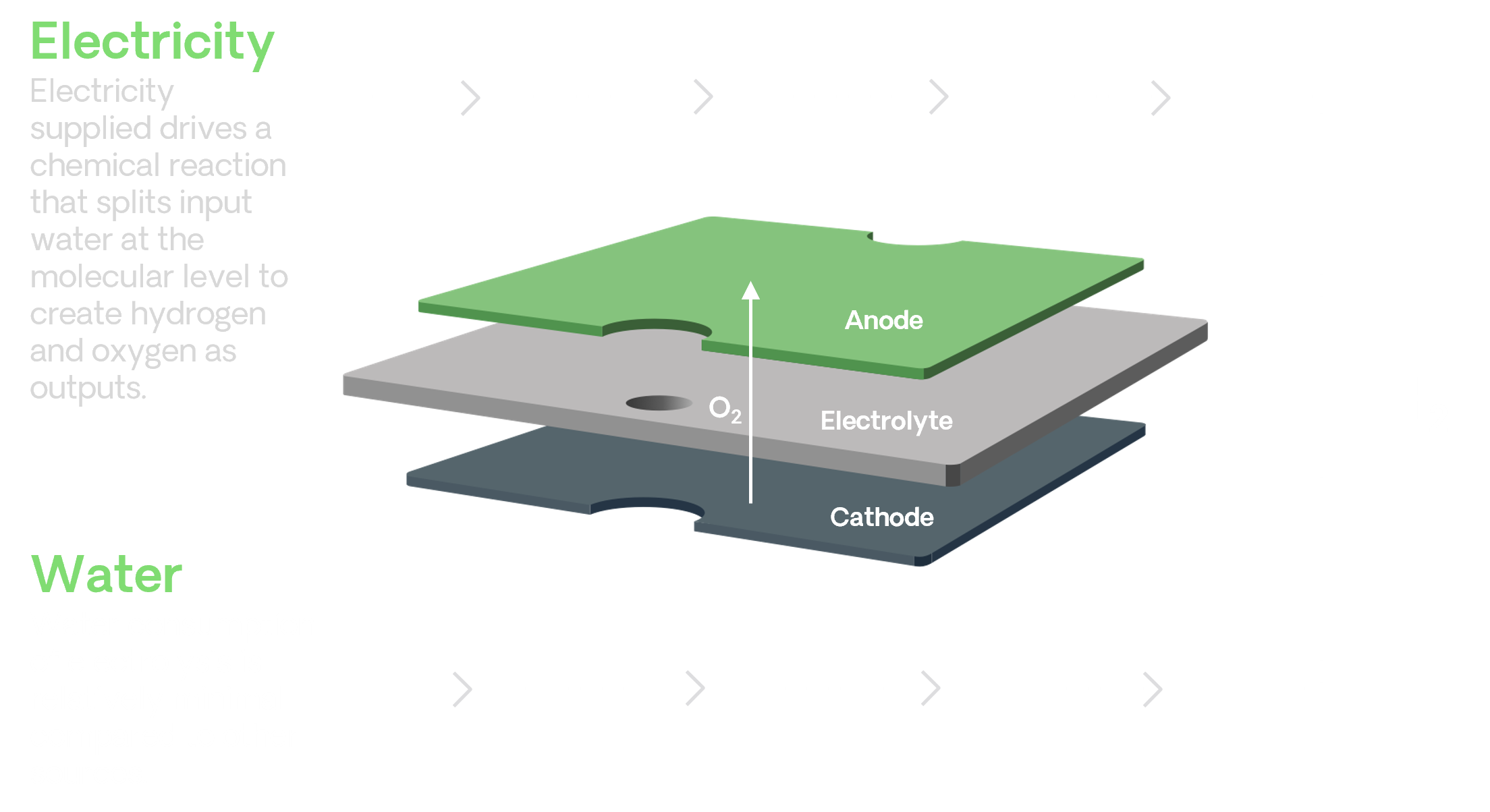
Electrolysis is a process that uses electrical energy to break water molecules into hydrogen and oxygen.
A solid oxide electrolysis cell (SOEC) consists of an anode, a cathode, and an electrolyte..
The electrolyte itself is a solid ceramic material, and the anode and cathode are made from special inks that coat the electrolyte and facilitate an electrochemical pathway to produce green hydrogen from renewable electricity.
High operating temperatures of solid oxide cells provide a greater overall efficiency than alternative technologies, thus requiring far less electricity to facilitate the reaction.


| PEM Electrolysis | Alkaline Electrolysis | Solid Oxide Electrolysis | |
|---|---|---|---|
| Description | Based on polymer membrane on a plate under high voltage and high current | Production reaction occurring in liquid alkaline solution | Solid ceramic material as electrolyte operating at high heat to reduce electrical needs |
| Materials Availability | Limited (PGMs) |
High | High (Robust supply chain) |
| Efficiency (kWh/kg) | 52 | 54 | 37-44 |
| Estimated learning rate | 13% | 9% | 28% |
| Developing | Mature | Mature |
Hydrogen is the clear answer for our changing energy needs. See why solid oxide technology is the most effective way to produce hydrogen and how Bloom Energy is leading decarbonization efforts across industries.
To get in touch with sales, inquire here
SAN JOSE, Calif., July 14, 2021 – Bloom Energy (NYSE: BE) today unveiled the Bloom Electrolyzer; the most energy-efficient electrolyzer to produce clean hydrogen to date and 15 to 45 percent more efficient than any other product on the market today.