Hydrogen is emerging as the clear choice to decarbonize industries like steel, transportation, and chemicals. Efficiency is key to scaling hydrogen as a viable renewable energy alternative. Of the technologies that can produce green hydrogen, solid oxide electrolyzers use energy inputs more efficiently, resulting in lower costs to produce hydrogen.
If you’re thinking about the benefits of hydrogen as an energy source, you’ve probably wondered at some point how expensive hydrogen is per megawatt (MW) of power. But the cost per megawatt varies depending on a variety of factors.
There is a better way to think about hydrogen created by electrolysis, the process by which electricity is used as a power source to split water into hydrogen and oxygen. A highly efficient electrolyzer such as the Bloom Electrolyzer reduces the number of megawatts needed to produce an equivalent amount of hydrogen. To put it simply, end-users get more hydrogen per megawatt of energy input with a more efficient electrolyzer. That means assessing the value of the electrolyzer by the capital cost per megawatt alone does not account for the added efficiency and long-term affordability.
The Secret to Hydrogen
As a long-time hydrogen project and business developer, I have spent many years in the industrial gas industry. I’ve developed large-scale hydrogen projects, managed hydrogen businesses, and developed and extended hydrogen supply agreements. Over the years I learned that people who consume hydrogen are interested in either how much volume or how much mass of hydrogen they are buying.
But here’s the secret: no one buys hydrogen by the amount of input energy or wattage. A comparison would be if you were to try making ammonia doing bright annealing or manufacturing renewable diesel—all of which require hydrogen intense processing—with megawatts. That’s not the right measure for those processes.
For those who have studied engineering, this goes back to when we learned about the units of measure on the first day of each class, and why different units are used as a standard measurement for certain quantities. Wattage measures the input unit used to produce the hydrogen that will supply your business. However, it fails to measure the overall energy savings and increased efficiency of producing hydrogen via an electrolysis technology that is more efficient than others—which shows up in the amount of hydrogen that is produced from a given amount of electrolyzers or electricity input to the system.
The Right Way to Measure Hydrogen
When considering hydrogen produced by electrolysis, I encourage stakeholders to think about not only the input wattage but, first, the quantity of hydrogen that will be produced.
Why is this important? Different electrolysis technologies such as solid oxide or polymer electrolyte membrane electrolysis (PEM) each present different balances of efficiency, capital cost, and degradation over time. Efficiency includes how much electricity the technology uses to produce hydrogen.
If you are a hydrogen user or even a hydrogen seller (as opposed to a seller of electrolyzers) the first question to ask is how many millions of standard cubic feet (MMSCFD), or kilograms, per unit of time is required. This gets at “how much hydrogen do I need”? Then evaluate the amount of electrolysis equipment that is required. Specifically, this will highlight the efficiency benefit: more efficient electrolyzers require less input electricity and less nameplate megawatts of electrolyzers required to make a given quantity of hydrogen. Less megawatts of electrolyzer capacity means lower operating and maintenance costs, which results in a compound favorable benefit beyond simply using less electricity to make a given hydrogen volume.
Here’s a high level example:
Let’s say your renewable diesel facility requires 30 millions of standard cubic feet of low-carbon intensity hydrogen and you want to evaluate electrolysis efficiency. What do you need in terms of electrolyzer capacity and how much electricity is required?
How Efficient is Hydrogen Production?
A Brief Demonstration
Let’s compare Bloom’s high temperature, solid oxide electrolyzer at 39 kilowatt hours (kWh) per kilogram (kg) of hydrogen versus an alkaline electrolyzer at 52 kWh per kg of hydrogen.
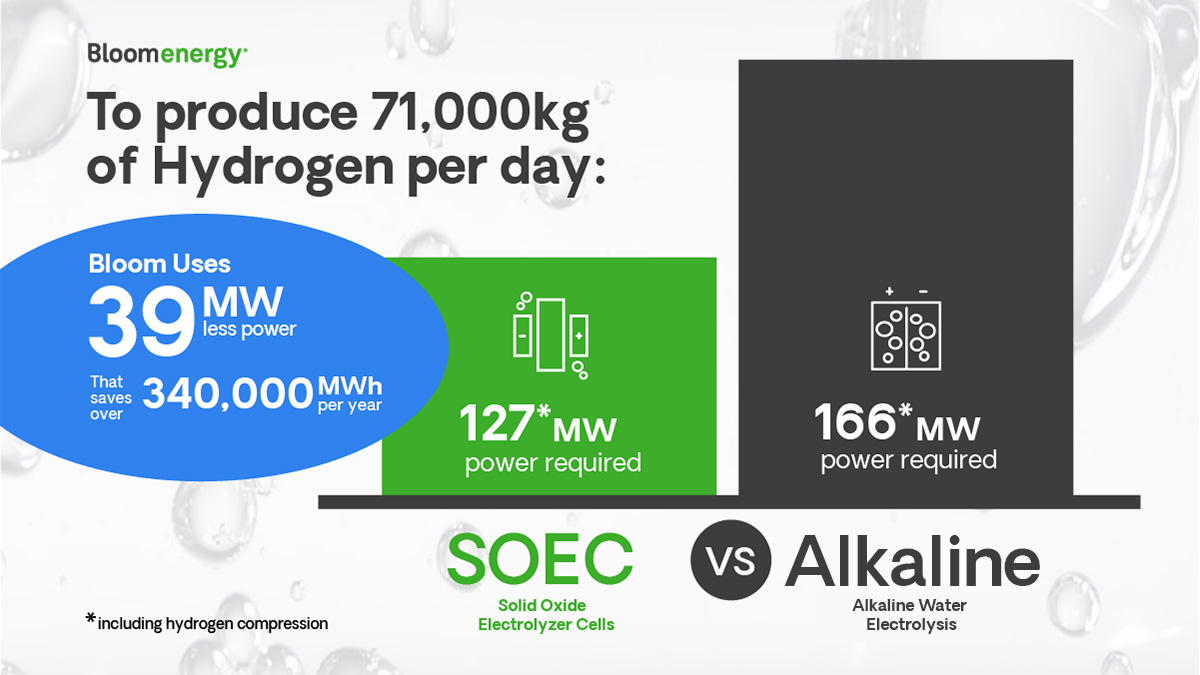
30MMSCFD of hydrogen converts to approximately 71,000 kg per day (using 423 standard cubic feet per kg).
At 39 kWh per kg with Bloom’s electrolyzer you need roughly 115 MW of electrolysis capacity. With the alkaline technology, you would need approximately 154 MW of electrolysis capacity. With Bloom, not only do you need to contract for less power, but you will also buy, install, and operate a lower capacity of electrolyzers.
Depending on the technology, hydrogen compression may be required. Both alkaline and Bloom’s solid oxide technology require hydrogen compression. Let’s assume 4 kWh per kg to achieve 300 pound-force per square inch. You would need to add 71,000 kg * 4 kWh/kg /24 = approximately 12 MW for each technology.
Therefore, to make high pressure hydrogen with the Bloom electrolyzer, you would design 115 MW of power for the electrolyzers, plus 12 MW of power for compression, totaling 127 MW of renewable power.
With alkaline technology, the design would be 154 MW of electrolyzers, plus 12 MW for compression, totaling 166 MW of renewable power.
What this Means for Customers
That means Bloom’s solid oxide electrolyzer can produce the same amount of hydrogen as other technologies but uses 39 megawatts less of input power. When converted to megawatt hours the amount of electricity saved amounts to over 340,000 megawatt hours a year less when using the Bloom electrolyzer.
Therefore, rather than asking how much an electrolyzer costs per megawatt, the first question to ask is: how much hydrogen do you need? Next, consider the differences in electrolyzer efficiency as shown above. From there, you can calculate how many megawatts of electrolyzers you need, before calculating about how much an electrolyzer would cost per megawatt over its lifetime.
Focusing on capital cost alone as a primary decision point leads to a higher capacity of inefficient electrolyzers, driving up the electricity needs, space, and leveled cost of hydrogen production. When you plug these facts into a discounted cash flow analysis, the answer quickly becomes apparent.
Of course, there is more to the story on hydrogen. I’m excited in the future to share thoughts that might be helpful on the importance of availability (like they say in the NFL, “the most important ability is availability”), degradation, solid oxide technology, and more. But at the foundation of everything is asking the right questions. That means carefully considering the efficiency of electrolyzers to meet your hydrogen needs. At Bloom Energy, we are proud to offer the most efficient answer to meet your hydrogen needs.
Bloom recently announced their first international electrolyzer deployment in South Korea. The electrolyzer, which is fully operational, showcases pathways to produce clean, low-cost hydrogen at scale to unlock South Korea’s net-zero future.
Hydrogen is a critical energy source for the transition to net-zero. With efficient electrolyzers, we can commercialize the production of sustainable hydrogen and advance our decarbonization goals today.